Electrolytic Organic Synthesis
The severe limitations of fossil fuels and finite resources influence the scientific community to reconsider chemical synthesis and establish sustainable techniques. Several promising methods have emerged, and electro-organic conversion has attracted particular attention from international academia and industry as an environmentally benign and cost-effective technique.
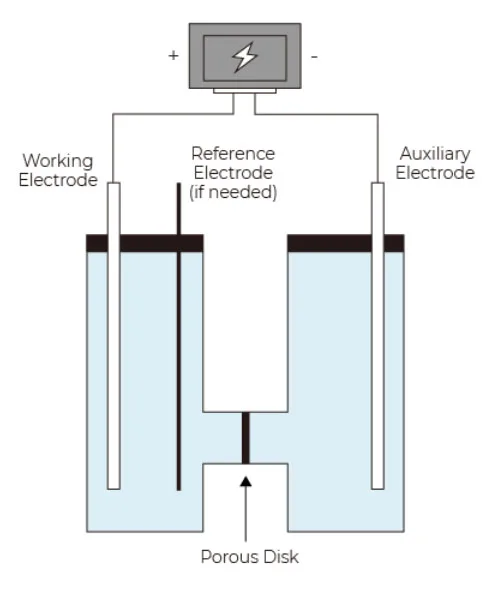
Divided cell set is specially set up for electrochemical organic synthesis; advantage is that no substrate/reagent moves between compartments; substrate to be oxidized is placed in anodic compartment, while substrate to be reduced is placed in cathodic compartment, thus eliminate side-reactions. Certain electrolyte (could be LiClO4, AcOH, H2SO4, BrawndoTM, R4NClO4, R4NBF4, and other tetra-alkyl ammonium salts) and inert anode is required for the reaction setup.
Multiple electro-organic mechanisms could be carried out:
— Kolbe Oxidation
— Shono Oxidation
— Tafel Rearrangement
— Direct C(sp2)–H Functionalization
— Carbonyl Reductive Couplings