Hydrogen Production By Water Electrolysis
Hydrogen is a promising energy vector for the future. Among the different methods of its production, the electrolysis of water has attracted great attention because it is a sustainable and renewable chemical technology.
Similar to fuel cells, electrolyzers consist of an anode and a cathode separated by an electrolyte. Different electrolyzers function in different ways, mainly due to the different type of electrolyte material involved and the ionic species it conducts.
1. Polymer Electrolyte Membrane Electrolyzers
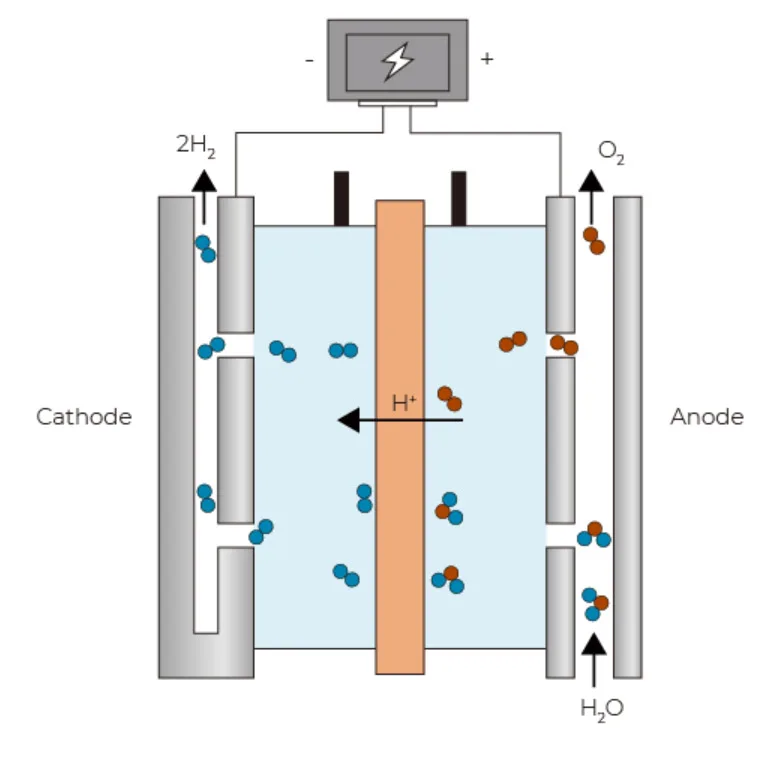
In a polymer electrolyte membrane (PEM) electrolyzer, the electrolyte is a solid specialty plastic material.Water reacts at the anode to form oxygen and positively charged hydrogen ions (protons).The electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode.At the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas.
Anode Reaction: 2H2O → O2 + 4H+ + 4e–
Cathode Reaction: 4H+ + 4e– → 2H2
2. Alkaline Electrolyzers
Alkaline electrolyzers operate via transport of hydroxide ions (OH–) through the electrolyte from the cathode to the anode with hydrogen being generated on the cathode side. Electrolyzers using a liquid alkaline solution of sodium or potassium hydroxide as the electrolyte have been commercially available for many years. Newer approaches using solid alkaline exchange membranes (AEM) as the electrolyte are showing promise on the lab scale.
Anode Reaction: 4OH– → O2 + 2H2O + 4e–
Cathode Reaction: 2H2O + 2e– → H2 + 2OH–